Nuclear Waste: Is it?
What is nuclear waste? Most people have no idea.
What is it?
The so-called waste atoms are contained in the spent, exhausted fuel cells after they come out of the reactor. They are the remains of the fuel's Uranium (U-235) and Plutonium (Pu-239) nuclei after they split (fission). These pieces are known as "fission fragments" in nuclear jargon. In everyday language, they are nuclear waste atoms.
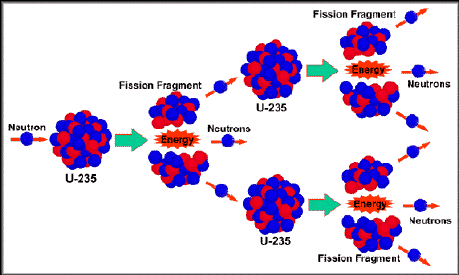
The resulting atomic fission products are newly-formed elements which are no longer U-235 or Pu-239. They have become a large number of common elements including all rare earths, a number of active metals, semi-precious metals and even a trace of Silver. Neutrons seldom spilt nuclei right down the middle. On uncommon occasions the U-235 and Pu-239 do split in half, but most of the time they don't. One thing that always happens; if you take the atomic number of one of the new atoms like Cesium (atomic no. 55), and subtract it from Uranium's atomic number (92), we get the atomic number of the other new atom, which is 37. This turns out to be Rubidium. Quite simply, the atomic numbers of the two new atoms, freshly made by splitting Uranium, always equal 92. When Pu-239 fissions, the atomic numbers of the two new atoms add up to Plutonium's atomic number of 94. Uranium and plutonium always split into two new atoms, and the resulting pairs are entirely predictable. As it turns out, The Cesium-Rubidium pair is the most probable result of U-235 fission, at a little less than 10% of all possible pairings. Other pairs in the 2% to 9% range include Barium-Krypton, Strontium-Xenon, and Yttrium-Iodine. The vast majority of pairings (always adding up to atomic number 92 or 94) are progressively less probable, all the way down to a tiny probability with the Iron-Dysprosium pair, at much less than .001 %. In total, a spectrum of 42 possible elements result from Uranium fissioning. With Plutonium, the pairings are slightly different because Pu-239 has a higher atomic number, which is two more than U-235. This adds four more possible elements, including Manganese, Chromium, Holmium and Erbium, all of which will have a spent fuel abundance of less than .01 %. In all cases, these freshly made elements are immediately radioactive. The most intensely radioactive waste elements are those with the shortest half-lives. The least radioactive waste elements are those with the long half lives. The ones we rationally need to concern ourselves with are those with half-lives between one day and five billion years. All radioactive materials will lose their detectable radioactivity after about nine half-lives. To be conservative, let's say the radioactive lifetime of a radioactive isotope is ten half-lives. Any isotope with a half life less than a day is essentially non-radioactive after 10 days, and is of no long-term consequence. It takes longer than 10 days after reactor shutdown to begin moving the used fuel out of the reactor for long-term storage, so the very short half-life isotopes are not an issue with spent fuel.
Numerically, about 5% of the waste elements made from splitting U-235 and Pu-239 have half lives less than one day. Another 75% of the radioactive elements we find in high level nuclear waste have half lives between one day and five years. We thus find the majority of the waste elements are non-radioactive after about 50 years, or ten half-lives. This includes many valuable rare earths such as Neodymium, (welders goggles, light-spectrum calibration equipment in astronomy, and laser technology) and Ruthenium (low cost solar cells). Other modern uses of these Rare Earths include magnets in hybrid cars, wind turbines, computer hard drives and cell phones. There are also many active, semi-precious metals in the waste atom matrix such as Cadmium, which is used in making batteries and electroplating. Recycling exhausted power plant reactor fuel after 50 years of closely-monitored storage would make these resources available to the world. Burying un-recycled fuel cells would be throwing these valuable materials away, which would be a true waste.
In fact, only 8 of the nuclear waste elements have isotopes which are of radioactive concern after 50 years.Of those, only 3 of the 15 Rare Earths in nuclear waste are detectably radioactive after 50 years (Promethium, Gadolinium, and Terbium). Recycling would remove these long-lived radioactive elements from the non-radioactive matrix and have them placed back into storage. But, should we just throw these remaining long-lived radioactive residuals away? Of course not, because of the 3 valuable Rare Earths and a bit of Silver in there (which has the longest isotopic half life of about 100 years), plus four other valuable active and semi-precious metals. Eventually, these useful materials can be recovered and become a valuable resource to our future descendants. Patience is, after all, a virtue.
In actuality, by making "nuclear waste atoms" we're literally realizing the old alchemist's dream of turning crude base metal into something precious. Split U-235 and Pu-239, recycle the spent fuel after 50 years, and we get lots of valuable stuff. As it turns out, by removing all of the so-called waste atoms from the exhausted fuel, and we do nothing other than bury the stripped fuel cells, the remaining fuel cells becomes less toxic than natural Uranium in less than 500 years! That's a long time, to be sure, but not the thousands-upon-thousands of years routinely expounded on through the news media and preached about by many governmental bodies. By not recycling the fuel, it remains more toxic than natural uranium for about 100,000 years. That's a very, very long time, but it need not be the case!
"It'll be radioactive forever!"
The BIG Problem...or is it?
Where did naturally-occurring U-235 come from, anyway? It doesn't seem to be found anywhere but in a Uranium deposits here on Earth. U-238 is sometimes found in cosmic rays but no U-235, and, many non-Uranium ores (such as coal) have trace amounts of U-238 but no U-235, indicating there is something strange about U-235 being found on Earth at all. Then the (above) discovery of natural Plutonium was made. Also, another remarkable discovery occurred. During nearly a half-century of military breeder reactors producing a significant stockpile of Plutonium, some of the concentrated Plutonium and Uranium-235 matrix literally sat around for decades awaiting its use in weapons. During that time, something strange and unexpected happened. The expected concentrations of Pu-239 and U-235 in the "old" stockpiles were off a tiny bit. There was a bit too much U-235, and a bit too little Pu-239. As it turns out, Pu-239 radioactively decays by releasing the nucleus of a helium atom (Alpha radiation) out of its nucleus, and becomes U-235! These two discoveries gave atomic scientists a possible reason for the existence of U-235 in nature.
When Earth formed, ~4.5 billion years ago, there must have been a primordial matrix of about 50% U-238 and 50% Pu-239 occurring, all uniformly mixed together. Pu-239 has a half-life of ~24,000 years. After about a quarter of a million years, all the Pu-239 had become U-235. U-235 has a half-life of ~700 million years. In the 4.5 billion years since the Earth was formed, the U-235 has undergone about 6.5 half-lives, resulting in the 0.7% abundance we find today. Thus, when we realized the existence of trace amounts of Pu-239 found in a few natural Uranium deposits, and the existence of Plutonium's daughter element U-235 in Plutonium stockpiles, we found that Plutonium is, was, and always will be a naturally-occurring element.
The "weapon's grade" myth
Yet, there is the stigma of the term "weapon's grade" that is routinely attached to power plant reactor-made Plutonium, which must be addressed. The existence of Plutonium in spent reactor fuel is widely braodcast as being "weapon's grade" by its very existence. There is literally never any opposiing perspective given. Let's ask a strange-sounding question. Is it really weapon's grade? Can power plant Plutonium actually be used to make a nuclear weapon?
When a new fuel cell goes into a large, relatively modern reactor it somewhere between 1% and 3% U-235, depending in the make and model of the fuel core. When a fuel cell leaves the reactor after it's 3-year "lifetime", there are almost no U-235 atoms left in it, but there is the 5% "waste" atoms and about 1% Plutonium found in its place. The 5% "waste" atoms tell an interesting story. With 1% U-235 fuel, only about 20% of them could have possibly come from U-235 fissioning. Further, 1-3% Uranium cannot make 5% waste atoms...the numbers just don't add up. Where did the rest of the waste atoms come from? Plutonium fissioning! While the initial loading of U-235 fissions, the U-238 is making Plutonium about as fast as the U-235 is being split. As it turns out, more than half of the energy each fuel cell makes during it's lifetime comes from the splitting of Plutonium. Further, the 1% Plutonium in the spent fuel coming out of power plant reactors is no more "weapon's grade" than the 1-3% U-235 encased in fuel that originally went into the reactor. Spent fuel containing Plutonium is no more "weapon's grade" than a brand new fuel cell before it goes into a reactor.
But, there's yet another problem with calling power plant Plutonium "weapon's grade". About a third of the Plutonium made in power plant reactors is not the Pu-239 used to make bombs. In fact, a third of the Plutonium isotopes in spent fuel bundles would make an explosion impossible!
Two isotopes Plutonium isotopes are being formed in power plant reactors: Pu-239 and Pu-240. Pu-240 atoms naturally experience two additional neutron absorbtions and become Pu-242, which radioactively decays by Alpha emission and becomes U-238, which is where it all started. The production of Pu-240 eventually plateaus at 0.32% abundance, and the Pu-239 at 0.68% abundance in the exhausted fuel cell. It's all good for reactor fuel. But, it's junk for bombs. A mixture of a 100% pure matrix of power plant Plutonium, at 68% Pu-239 and 32% Pu-240, can never explode because Pu-240 doesn't fission. Further, Pu-240 absorbs neutrons better than Pu-239. As a result, Pu-240 is what might be correctly termed a "bomb poison", because it absorbs neutrons sufficiently to keep the chain reaction from becoming extreme enough for an explosion. All by themselves, these two reasons are why power plant reactor Plutonium can not be correctly termed "weapon's grade". Together, they make the term "weapon's grade" grossly inappropriate for power plant Plutonium.
Thousands of years, and no carbon footprint
Uranium is mostly useless to humans other than in reactors, armor for mechanized military vehicles, and bombs. It was historically used for ballast for large sailboats, but not any more. Plutonium is only useful for reactor fuel and bombs. We could say that every nucleus of U-235 and Pu-239 split in a reactor is one less atom potentially used in a bomb. Let's get rid of these bomb-possible isotopes and turn them into valuable resources (the "waste" atoms) that cannot be made into bombs. Let's utilize a paradigm of appropriate environmentalism and recycle (reprocess) exhausted power plant fuel cells. 95% of each exhausted fuel cell from a power plant reactor is still good fuel. By doing the environmentally appropriate thing, and recycling (reprocessing) the spent fuel from power plant reactors (after ~50 years?), we not only reclaim the valuable non-radioactive resources from the waste atom matrix, but we have a useful Uranium-Plutonium matrix for the making of new reactor fuel cells. Without recycling spent fuel, and only using each fuel cell once before discarding it forever as trash, we might have ~150 years of U-235 before we run out. Remember, U-235 is a trace isotope found in nature. However, by recycling the exhausted fuel, that useful lifetime stretches out to thousands of years…with no carbon footprint from the making of our electricity!
Think about it…
Summation:
1. The "waste atoms" in the fuel are approximately 46 newly-formed elements.
2. Most of the "waste atoms" can be realized as valuable resources through recycling.
3. Recycled reactor fuel can extend the availability of Uranium as a fuel by thousands of years.
4. Power plant Plutonium cannot be used to make a nuclear weapon.

References :
- Nuclear; Homeland Security News : National Terror Alert;
http://www.nationalterroralert.com/nuclear/; 2009 - Hvistendahl, Mara; Coal Ash is More Radioactive than Nuclear Waste; Scientific American; Dec. 13, 2007;
http://www.scientificamerican.com/article.cfm?id=coal-ash-is-more-radioactive-than-nuclear-waste - Gabbard, Alex; Coal Combustion : Nuclear Rescource or Danger?: Oak Ridge National Laboratory; Communications and External Relations Group; http://www.ornl.gov/info/ornlreview/rev26-34/text/colmain.html
- Backgrounder on Radioactive Waste; United States Nuclear Regulatory Commissionhttp://www.nrc.gov/reading-rm/doc-collections/fact-sheets/radwaste.html; August 18, 2009
- Whitlock, Dr. Jeremy; Waste Management; Canadian Nuclear FAQ;
http://www.nuclearfaq.ca/cnf_sectionE.htm; 2009 - Joint Convention on the Safety of Spent Fuel Management and on the Safety of Radioactive Waste Management; International Atomic Energy Agency; Vienna, Austria;
http://www-ns.iaea.org/conventions/waste-jointconvention.htm; 2003-2010 - Table of Nuclides: Korea Atomic Research Institute;
http://atom.kaeri.re.kr/; 2000 - Periodic Table of the Elements; Los Alamos National Laboratory's Chemistry Division;
http://periodic.lanl.gov/default.htm; updated,12/11/2003 - Chart of Radionuclides; Columbia University Department of earth and Environmental Sciences;
http://eesc.columbia.edu/courses/ees/lithosphere/labs/lab12/radionuclides/index.html - Interactive Chart of the Nuclides; Brookhaven National Laboratory; http://www.nndc.bnl.gov/chart/reCenter.jsp?z=6&n=8
- The Workings of an Ancient Nuclear Reactor; Scientific American, January 26, 2009. http://www.scientificamerican.com/article/ancient-nuclear-reactor/
- Montgomery, Jerry, Phd and Rondo, Jeffery, PhD; Asymmetrical Fission Products; Unclear 2 Nuclear;
http://www.unclear2nuclear.com/asymFission.php; 2008 - Fission Fragments; Georgia State University Department of Physics;
http://hyperphysics.phy-astr.gsu.edu/Hbase/nucene/fisfrag.html; 2006 - Boyd, Rex; Radioisotopes in Medicine; World Nuclear Association;
http://www.world-nuclear.org/info/inf55.html; updated, February, 2010 - Winter, Mark; Plutonium; Web Elements, Ltd.; University of Sheffield, England;
http://www.webelements.com/plutonium/isotopes.html; updated, 2009 - Plutonium; Department of Radiation Protection; U.S. Environmental Protection Agency;
http://www.epa.gov/radiation/radionuclides/plutonium.html; February, 2009 - Cohen, Bernard, L.; Plutonium and Bombs: Chapter 13 of The Nuclear Energy Option; Plemum Press Inc.; 1990;
http://www.phyast.pitt.edu/~blc/book/chapter13.html - Radioactive Waste Management; World Nuclear Association; August, 2015. http://www.world-nuclear.org/info/Nuclear-Fuel-Cycle/Nuclear-Wastes/Radioactive-Waste-Management/
Next - Three Mile Island https://www.hiroshimasyndrome.com/three-mile-island-and-the-china-syndrome.html