Background Information on Tritium
Background Information on Tritium
Tritium is the radioactive form of Hydrogen and one of the numerous radioactive isotopes produced by Mother Nature. Natural Tritium is caused by upper-atmospheric collisions between energetic molecules and cosmic rays. It is also formed by neutron bombardment of the water flowing through nuclear power plant reactors. Tritium has one proton in its nucleus, which makes it Hydrogen. But, it also has two neutrons attached to the proton, which is why it is symbolized as H3. The two neutrons cause the nucleus to be a bit unstable. The resulting radioactive process goes like this - one of the neutrons disgorges a weak electron, turns into a proton, and the Tritium instantly becomes inert, non-radioactive Helium (He3). The freed electron is called a Beta (β) particle, incorrectly dubbed a “beta ray” by the Press. Gamma and X-ray radiations are “rays” because they are a continual flow of energy. Beta radiation is negatively-charged sub-atomic particles…not rays.
Beta radiation has significant natural limitations. The most energetic Betas known to man (~7 Mev and up) cannot penetrate heavy-duty aluminum foil. Tritium’s Beta is the weakest produced by the radioactive isotopes formed inside nuclear power plant reactor cores (~18 KeV) – nearly four hundred times weaker than the above-mentioned high-power Betas. As a result, Tritium’s Beta can be stopped by cheap, thin cellophane. (1) In fact, as reported by Idaho State University’s Radiation Information Network, “The radioactive decay product of tritium is a low energy beta that cannot penetrate the outer dead layer of human skin.” (2) For the record, Tritium’s beta is stopped by 2-thousanths of an inch of water. It is very, very weak.
There is no scientific or medical evidence of Tritium actually causing cancer. The current Tritium limit was set in 1976 by the EPA based on… well… it is hard to say what it was based on. A recent article in Scientific American says that the current EPA limits were never based on health standards. Instead, the report says that the EPA “back-calculated” from nuclear weapon’s testing in the Pacific, which ended in 1963. (Tritium is added to the weapons to amplify the detonation) David Kocher of the Oak Ridge Center for Risk Analysis has studied Tritium for three decades, and told S-A, “It's not a health-based standard, it's based on what was easily achievable." America’s limit of 740 Becquerels per liter was chosen because "no drinking water anywhere was anywhere close, so it cost nothing to meet." (1) There is no record of the “back-calculated” methodology used to set the limit.
In 2006, the state of California ran an intensive study on Tritium and concluded that the EPA and NRC-imposed limits on Tritium in water are based on mere assumption…a hunch. In the case of Tritium, the original EPA limits weere literally a shot in the dark! California found that the only evidence for any negative health effects came from exposing lab mice to enormous levels of Tritium, in excess of 37,000,000 Becquerels per liter! While some specimens grew tumors, none of them were fatal. The report says, “Apparently, animals were not dying from tumors associated with tritium exposure.” The California report also states, “We have found no human data that specifically addresses the carcinogenic effects of Tritium.” Regardless, the California state limit on Tritium in water was adopted directly from the US national standard of 20,000 pci/liter (740 Bq/l) set by the EPA. Drinking two liters per day at 20,000 pci/liter was assumed by the EPA to result in an annual internal exposure of 0.04 millisieverts per year.
The following chart was supplied by colleague Tony Brooks. (4) It compares the levels of actual observable harm with Tritium exposure, regulatory limits found around the world, and the typical Tritium concentration of 7 Bq/l found in most water supplies…
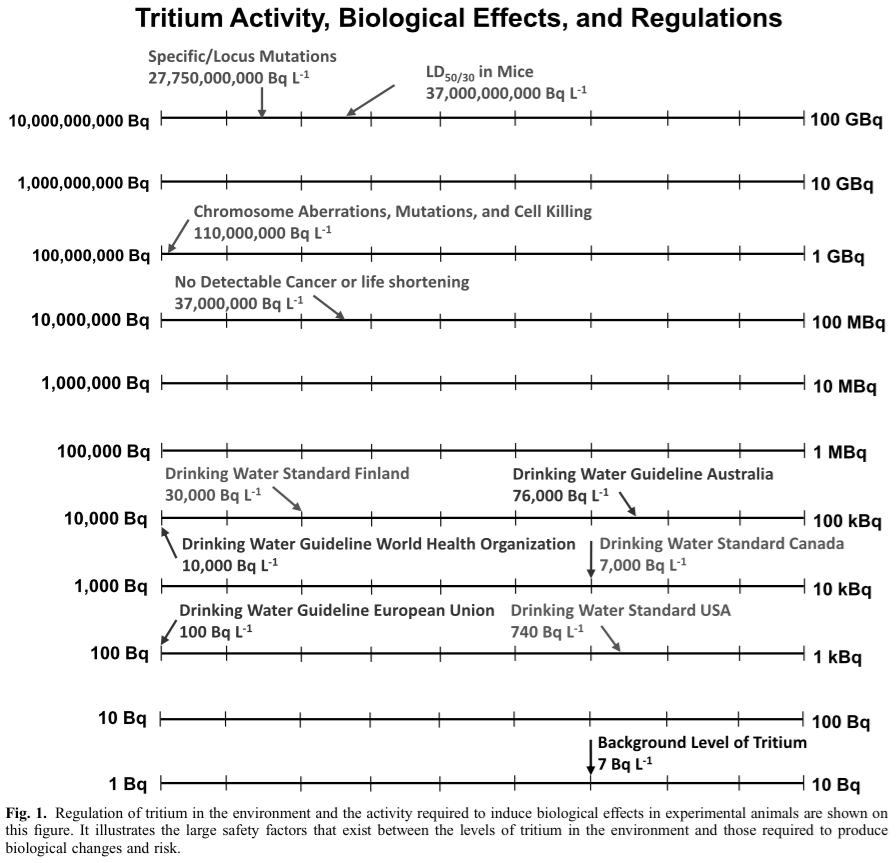
No fatal cancers have ever been observed below 1500 mSv/yr in humans due to any form of ionizing radiation, let alone Tritium’s weak beta. With Tritium’s extremely low energy beta, no rational correlation to the apparent 1,500 mSv/yr cancer threshold should be drawn. It should be noted that Canada’s limit is 7,000 Bq/l (5) and Japan’s standard for release is 60,000 Bq/l. Thus, the US and California limits on Tritium are ultra-conservative. In fact, we might correctly judge them to be arbitrary and absurd.
To add more fuel to the mix, we might refer to a position statement on radiation exposure from the Health Physics Society’s fact sheet on Tritium, “While it has been determined that exposure to high levels of ionizing radiation causes cancer, this effect has not been observed for lower doses on the order of background radiation doses, including with tritium. Although the regulatory process and radiation safety practices operate based on the conservative assumption that all radiation exposure increases cancer risk, credible quantitative risk assessments cannot be made for low background levels of radiation exposure. For this reason, the cancer risk, if there is any, posed to a member of the general public by expected environmental tritium exposure levels cannot be determined reliably. In fact, it has been shown that the health effects, namely cancer induction, resulting from such exposures are too improbable to be observed using current data and analytical methods.” (6)
As it turns out, the alleged connection between Tritium exposure and cancer (or genetic damage) also seems to be an assumption based on the mere fact that Tritium is radioactive. The EPA assumes that if something is radioactive, then it must be a cancer risk, regardless of evidence to the contrary. The EPA admits it in their fact sheet on Tritium, “As with all ionizing radiation, exposure to Tritium increases the risk of developing cancer.” (7) The posting says nothing about how this assumption was reached, and no evidence is given to support it. We find confirmation on the EPA’s vacuous assumption in the Nuclear Regulatory Commission’s “backgrounder” on Tritium. (8) The backgrounder says the Tritium limit in drinking water of 740 Bq/liter was “assumed to yield a dose of 4 mrem per year” (0.04 millisieverts/year). However, in 1991 the EPA re-calculated on their assumption and found the 0.04 mSv/yr exposure would require the daily intake of at least 2 liters of water containing 2250 Bq/liter of Tritium, not 740 Bq/liter. But, the EPA decided not to raise the limit, and the NRC went along with it.
There is no conclusive evidence of a connection between Tritium’s extremely weak beta and cancer incidence. It appears to be purely conjectural. There are many reputable references which say Tritium can cause molecular ionization; after all its Beta is an electron and adding or removing electrons from atoms is ionization, by definition. Thus Tritium can hypothetically cause ionization of molecules in our DNA, although unlikely because the electron would probably be swept up by other cellular molecules before it ever reached a nucleus. Here’s the bottom line… there is no scientific or medical record of Tritium actually causing DNA damage, cancer, or inheritable mutations, even with the massive, literally unthinkable concentrations given to lab animals. Thus, it seems the standards on Tritium exposure are entirely arbitrary, predicated on assumption, and devoid of actual evidence.
But, what about Fukushima and its hundreds of thousands of tons of waste water containing ~630,000 Bq of Tritium/liter? What if all the tanks holding the wastewaters suddenly failed and poured their entire contents into the Pacific Ocean? Although Tritium releases to the sea literally pose no discernable risk, Japan appears poised to do whatever it takes to keep the discharges to a minimum at an enormous cost.
Early in 2014 the Tokyo Electric Company began the full-scale operation of their Advanced Liquid Processing System (ALPS) at Fukushima Daiichi. It removes the 60+ radioactive isotopes remaining in the currently-stored wastewater that has been run through the Cesium absorber system. ALPS uses high-efficiency resin beds to strip the materials from the liquids flowing through them. Tritium is the only radio-isotope that remains. (9) By the end of 2015, 98% of all Fukushima waste water had been treated for reduction of all radioactive isotopes, except for Tritium.
The ultimate question will be whether or not Tepco will be allowed to release the ultra-clean, Tritiated water into the sea to mitigate their wastewater storage problem. Any detectible radioactivity makes millions of Japanese fear for their lives. The mere thought of eating seafood containing miniscule, non-detectible amounts of radioactivity makes many consumers shun fish caught off the Fukushima coast. The fisheries of Fukushima have declared that they will never agree to allow Tritiated water releases to the sea because unfounded rumors will hurt their business. Why not just rip the little atom out of the water. Wouldn’t that solve the problem?
Because it is hydrogen, Tritium is part of the water molecule. This is why it cannot be removed by Fukushima’s Cesium absorption units, reverse osmosis system (to reduce salt concentration), or ALPS resin beds. The existing systems cannot remove water from water, if you will. Separating Tritium from non-radioactive water is possible, but it is a slow and expensive process. The best methodology is about 90% effective, at most. There are international markets for Tritium including biomedical research and some glow-in-the-dark technologies, such as emergency exit signs and remote-location airport runway lights. In all cases, the technologies produce less radiation exposure than household smoke detectors. The advantage is that the Tritium-based equipment needs no power to operate and never stops working. While building a Tritium removal facility at F. Daiichi for international marketing might seem a reasonable solution, the financial investment would be massive and the demand for the Tritium produced would be limited. Besides, Tepco doesn’t have the money for it. The government would probably have to create a subsidy before it would happen. Even then, this might not be good enough to assuage Japan’s substantial radiation-paranoiac demographic. To them, the only acceptable release will be no release.
Tepco cannot build more and more wastewater tanks ad infinitum. At some point, the stored-water buildup must be curbed. Inevitably, the purified, Tritiated waters will have to be released into the ocean. As stated by expert consultants Lake Barrett, Dale Klein, and Lady Barbara Judge, there is no other option. But, that isn’t the point. Tritium has never been found to actually cause cancer in any human being or lab animals massively super-gorged with the stuff. There is one case of a woman accidently ingesting 37 billion Becquerels of Tritium, resulting in detectible chromosomal aberrations for 11 years - all of which were repaired by her body’s natural mechanisms – but no cancer or genetic damage. (NCRP, 1979) Other than this, no negative health effects in humans due to Tritium have ever occurred. However, both the US EPA and NRC assume that because Tritium is radioactive, and assume all radiation causes irreparable damage to living cells and DNA, they subsequently assume that any Tritium exposure via ingestion necessarily places people at an extremely low level of risk.
Using the above facts as background, we can look at the Tritium levels in the wastewaters at F. Daiichi and reasonably evaluate whether or not it will be worth getting upset about. The Tritium activity level at F. Daiichi is 630,000 Bq/liter in samples taken from the untreated turbine building basements. There’s no reason to think the waters inside any of the storage tanks at F. Daiichi have higher concentrations. After ALPS does its job, let’s make the worst-case assumption that 630,000 Bq/liter of Tritium will remain in each tank. Now, let’s make a ridiculous assumption and say someone drinks the waste water, raw and undiluted. The realm of exposure for adverse, non-lethal, biological effects in humans seems to be 37,000,000 Becquerels. This means, someone would have to drink about 14 gallons of the undiluted Tritiated waste waters at Fukushima in order to be non-lethally harmed.
How probable is that?
As we can see, once all the waste water at Fukushima Daiichi has been stripped of everything except Tritium they could pump it directly to the sea and no person or sea creature would be harmed. But, it seems Japan will follow a recent IAEA suggestion and dilute this harmless level of Tritium to well-below Japan’s limit of 60,000 Bq/liter before releasing it. Along the way, the Press will make a mountain out of this imaginary mole-hill, Japan’s antinuclear demographic will scream bloody-murder, and the international prophets of nuclear energy doom will guarantee widespread cancer epidemics along the Pacific Rim. All because of a low energy form of radiation that wouldn’t hurt a mouse!
* The NCRP report, "Tritium in the Environment", Report no. 062, is behind a paywall. It can be found here - https://ncrponline.org/shop/reports/report-no-062-tritium-in-the-environment-1979/ The report is central to the "Public Health Goal for Tritium in Drinking Water" posted in 2004 by the Office of Environmental Health Hazard Assessment California Environmental Protection Agency. The report can be accessed here... https://oehha.ca.gov/media/downloads/water/public-health-goal/drafttritium.pdf
Other references:
2 - BROOKS, ANTONE L., COUCH, LEZLIE A., and CHAD, SCHAEFER A.; COMMENTARY: WHAT IS THE HEALTH RISK OF 740 BQ/ L OF TRITIUM? A PERSPECTIVE; Health Physics Society; 2012
3 - http://www.cna.ca/nuclear_facts/safety/tritium-info/
4 - http://hps.org/documents/tritium_fact_sheet.pdf
6 - http://www.nrc.gov/reading-rm/doc-collections/fact-sheets/tritium-radiation-fs.html
7 - http://www.tepco.co.jp/en/nu/fukushima-np/handouts/2013/images/handouts_130329_01-e.pdf